The proteasome: an ATP-driven molecular machine for protein degradation
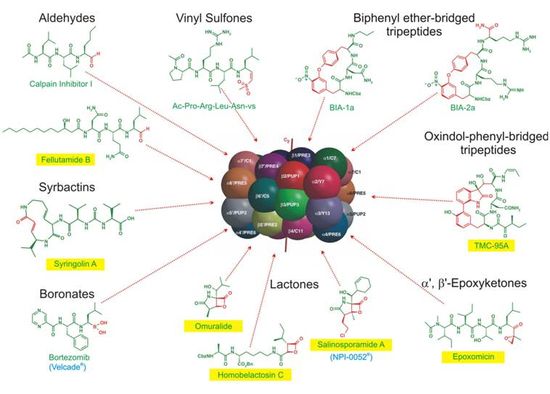
In eukaryotic cells most cytosolic and nuclear proteins are degraded by the ubiquitin-proteasome pathway. This pathway is responsible for many essential intracellular functions such as protein quality control, antigen processing, signal transduction, cell cycle control, cell differentiation and apoptosis. Furthermore, protein degradation is a major part of the life cycle of proteins, thus, destruction of proteins from their folded stage to single amino acids is a strictly controlled multistep process. In order to selectively degrade damaged, misfolded, or misassembled proteins, the eukyryotic cell carries out an enzymatic reaction modifying these substrate proteins with a labeling ubiquitin chain. Once ubiquitinated, the proteins are recognized and rapidly degraded to small peptides by the main proteolytic component of the ubiquitin-proteasome degradation pathway, the 26S proteasome complex. This ATP-driven multifunctional complex, having a molecular mass of approximately 3.000.000 Da and being assembled of more than 70 protein subunits, can already be regarded as a small “molecular” organelle, acting as a highly sophisticated protein destruction manufactory. Assembly, architecture, mechanism, function and regulation of the 26S proteasome are only poorly characterized and are nowadays of increasing interest in active research. Furthermore, natural and synthetic products, selectively inhibiting or modulating proteasomal activity would imply great biological and biomedical significance, thus, being highly appealing for a large variety of companies. The proteolytically active proteasome core complex has been already established as a promising target in cancer therapy with Bortezomib, as a first commercial inhibitor available on the market as a prescriptive first-line treatment against multiple myeloma. This is one of the only two drugs, together with the natural product Salinosporamide A that have passed clinical studies and found its practical application in medicine. Aside from these compounds, there are many other natural and synthetic products identified to be inhibiting this promising target and which need additional improvement. In contrast to traditional methods, the combination of crystallographic knowledge and organic chemical synthesis opens new horizons for drug design strategies, producing new potential lead compounds with a more economic and simple chemical synthesis. This proposed interdisciplinary approach brings coexistence of academic science and pharmaceutical industry on a new advanced level and contribute to the further understanding of this sophisticated pathway.