Polymer-based Hybrid Solar Cells
Prof. Dr. Peter Müller-Buschbaum
A very promising approach in organic photovolatics is based on the so called hybrid photovoltaic cells (see figure), which are a combination of two independent solar cell technologies: the dye-sensitized solar cell (DSSC) and the polymer solar cell consisting merely of p- and n-type conjugated polymers.
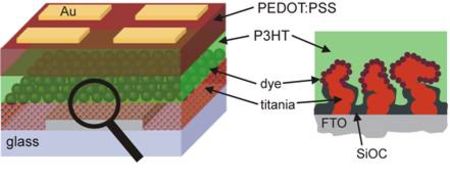
The DSSC consists of dye-sensitized nanostructured titanium dioxide on a compact transparent semi-conducting oxide (titania) and the multi-component redox-electrolyte filled in between the two electrodes. Although they are still some of the most efficient organic solar cells, the presence of the liquid electrolyte creates sealing issues which also initiated the research for an alternative hole- conducting material. For the hybrid photovoltaic cells the electrolyte is replaced by a p-type conducting polymer acting as hole-conducting and electron-blocking layer of the device. This combination of the inorganic semiconductor and a semi-conducting polymer provides the practical advantages of the organic material, e.g. synthetically tailored properties, simple processibility, mechanical flexibility, as well as the high electron mobility of the inorganic material. The performance of these hybrid devices significantly depends on the morphology of the nanostructured inorganic material, i.e. titania, because the morphology determines the volume-to-surface ratio and hence the surface available for interfacial reactions. Moreover, the morphology influences charge carrier transfer routes and thus electron-hole recombination probabilities. Therefore, the tailoring of the desired morphology on the nanoscale, yielding an efficiently performing hybrid solar cell, is of great importance and considered to be the crucial step of this type of device.
Using a sol-gel approach, the inorganic material is nano-structured. The type of inorganic structure, such as foam-like, nano-wire type or cauliflower-like, will have a strong influence on the performance of a potential device. Its back-filling with the polymer is still one of the great challenges. These imperfections have to be overcome in future work by a control of the surface chemistry of the titania, by different back-filling mechanisms or by super-structuring of the titania. All these different approaches are studied.