On-Chip Functionalization of Carbon Nanotubes by the Photosystem I
Prof. Dr. Alexander Holleitner
Photosynthesis in plants and bacteria is driven by molecular electronic complexes, such as the photosystem I (PSI). Photoexcitation of PSI causes an electron transfer through a series of redox reactions from the chlorophyll special pair P700, as a primary electron donor, to a final electron acceptor, ∼6 nm away from the oxidant P700+. Here, we used carbon nanotubes (CNTs) as nanoscale electrical wires to electrically contact the PSI [1,2]. We compared the optoelectronic properties of the CNT-PSI hybrids for three different on-chip functionalization strategies [3]. The PSI was bound to the CNTs via covalent, hydrogen, or electrostatic bonds.
For device fabrication, individual carboxylated CNTs are first deposited on an insulating SiO2 substrate and then contacted with Pd source-drain electrodes by e-beam lithography. In the first chemical route, selectively generated cysteins on the lumenal side of the PSI reacted with maleimide functionalized CNTs. AFM images before and after functionalization of the CNTs with PSI provide a first indication that PSI proteins are attached to the sidewalls of the CNTs due to covalent bonding between the CNT and the PSI. Hence, the electron transfer path in PSI is expected to be perpendicular to the CNT (sketch in Figure 2a). Filled squares in Figure 2a depict the wavelength dependence of the photoconductance of sample A, which was fabricated by the first chemical route. The photoconductance showed an enhanced signal at 680 and 360 nm. The findings are consistent with the absorbance data of the PSI (solid line in Figure 2a). Importantly, the enhanced GPH below 700 nm can be attributed to an effect of the PSI. This was proven by measurements on sample B (Figure 2b). Here, the photoconductance of a CNT bundle before and after on-chip functionalization with PSI by covalentbonding was compared. The maxima at 680 nm and at ∼360 nm only appear when the PSI is attached to the CNTs. Above 700 nm, the PSI does not absorb light, and in turn, the magnitude of the photoconductance before and after functionalization with PSI is the same within the experimental error. Consequently, the two photoconductance maxima around 760 and 845 nm and also the maximum at 825 nm in Figure 2a are interpreted to result from electron-hole dynamics only within the contacted CNTs [2,3]. These findings are consistent with resonances assigned to the E22 transition in semiconducting CNTs with a diameter in the range 1-2 nm [4]. The origin of the enhanced photoconductance at the resonances of the PSI can be attributed to an electron or energy transfer between the excited PSI and the CNTs.
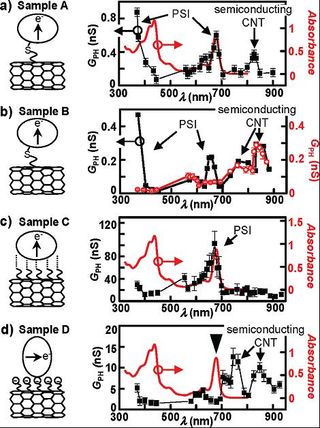
In the second chemical route, the electrically contacted CNT bundle of sample C was functionalized with ethylenediamine before the reaction with the PSI. AFM measurements proved that the PSI is adsorbed to the CNTs. This can be explained by hydrogen bond formation or electrostatic interactions between the PSI and the amino groups on the CNTs. In both cases, the PSI was preferentially orientated with its electron transfer path perpendicular to the amine modified CNTs (sketch in Figure 2c). The photoconductance of sample C showed a peak at 680 nm (Figure 2c) which again can be assigned to a charge or energy transfer between the PSI and the CNT.
In the third chemical route (sample D), the functionalization of the CNTs with PSI was enabled by electrostatic forces between negatively charged terminal groups on the CNTs and positively charged regions on the lumenal and stromal side of the PSI. AFM measurements demonstrated a partial adsorption of PSI to the CNTs. The wavelength dependent photoconductance of sample D (squares in Figure 2d), however, showed only resonances above 700 nm which are caused by electron-hole dynamics in semiconducting CNTs. Most importantly, there was no photoconductance resonance at 680 nm (triangle in Figure 2d), a fact which can be explained by a less efficient adsorption of the PSI to the CNTs and by considering the mechanism of adsorption of the PSI on the CNTs. Electrostatic interactions between negatively charged COO- groups and the PSI favor a parallel orientation of the PSI’s electron transfer path with respect to the CNT. For this alignment the photogenerated dipole is parallel to the CNTs’ axis and the proposed mechanism of energy transfer is less efficient. In addition, a possible charge transfer between the PSI and the CNT is suppressed in this configuration [2,3].
This work is supported by the DFG via Grant HO3324 and the Nanosystems Initiative Munich (NIM).
[1] I. Carmeli, M. Mangold, B. Zebli, L. Frolov, C. Carmeli, S. Richter, A.W. Holleitner, Adv. Mat. 19, 3901 (2007)
[2] S. M. Kaniber, F. C. Simmel, A. W. Holleitner, and I. Carmeli, Nanotech. 20, 345701 (2009)
[3] S. M. Kaniber, M. Brandstetter, F. C. Simmel, I. Carmeli, and A. W. Holleitner, J. Am. Chem. Soc. 132, 2872 (2010)
[4] S. M. Kaniber, L. Song, J.P. Kotthaus, and A.W. Holleitner, Appl. Phys. Lett. 94, 261106 (2009)