Experimental Nuclear Astrophysics at TUM
The nuclear astrophysics group at the Technische Universität München welcomes you to our web portal.
Nuclear Astrophysics
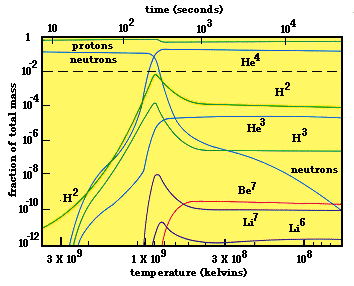
Experimental nuclear astrophysics is largely concerned with the business of element production; that is, Nucleosynthesis. What we know of our Universe is that, within the first few minutes of its popping into being, only the lightest elements, ranging from simple hydrogen up to beryllium, were produced. You can see this in the plot below which shows, as a function of time since the beginning of the Universe (along the top horizontal axis) and temperature of the nascent Universe (along the bottom horizontal axis) the mass-abundance fractions of these light elements. After some few thousand seconds, the abundance fractions no longer change with time (or temperature), signalling the end of element production. (The ongoing decrease in neutrons is due to their beta-decays into protons. This process is not nucleosynthesis). The production of these light elements within the first few minutes of cosmic history is called Big Bang Nucleosynthesis (BBN).
The figure above also shows how boring our Universe could have been. By far, the most abundant elements from BBN are just protons (hydrogen) and helium (4He). Alll others are a factor of 10 000, or more, smaller in abundance than these two; what an uninteresting chemistry such a Universe would have! Hydrogen would be locked up as molecular H2 and helium, a Nobel gas, is chemically inert! Very little chemistry could happen in such a Universe.
Yet, we are here; we exist. And surrounding us on a grand scale is a veritable plethora of chemistry: from the intricate chemical "factories" churning away inside every living cell on our planet, the photosynthesis that makes sunlight into sugar, to the life-enabling organic molecules found in the dust corridors between the stars.
To Greater Complexity: Chemical Evolution
How did the chemistry of the Universe evolve from its humble beginnings above, with just 4 elements, to that of which we find around us: the oxygen we breath, the calcium in our bones, the iron in our blood? While we utilize uranium in nuclear reactors for power, and harvest gold from the deep earth for jewelry, and consider just how precious, rare and expensive these two elements are, we again ask: from where did these originate during the evolutionary course of our Universe, and why are their abundances so low compared to iron or other common metals? Indeed, why are the elemental abundances what they are?
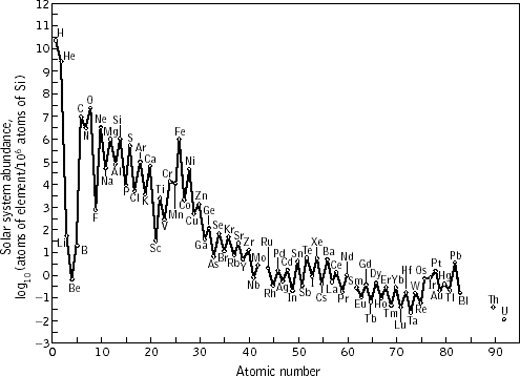
Above, and in contrast to the first image above, is a plot of the elmental abundances respresentative of our solar system. These are determined from precision measurements of atomic line emission/absorption in our Sun's atmosphere and from isotopic measurements of material contained in meteorites that fall to Earth. Here, we see the presence of all elements from hydrogen up to uranium. We also see that the abundances themselves span about 11 orders of magnitude from hydrogen to tantalum (Ta) and uranium (U). We also see structure in the plot: there is an oscillatory "up and down" of the abundances in going from those with even mass number A to odd mass number. We also see an abundance peak around iron (Fe), from which the abundances suddenly drop by about 6 orders of magnitude until reaching a smaller peak around xenon (Xe) and then finally another secondary peak around lead (Pb).
For the nuclear astrophysicist, knowledgeable of nuclear physics and the inner workings of stars, the "fingerprints" of nuclear phenomena and those of the extreme internal conditions within stars are all over this plot. That iron and nickel are the two nuclei with highest binding energies is partly the reason for the iron peak. That the binding of nuclei depends on the eveness or oddness of the total number of nucleons provides the first clue to the oscillatory behaviour of the abundances. Nuclear shell structure plays a large role in explaining the two secondary abundance peaks at Xe and Pb. And the fact that the abundances drop off so radidly between carbon (C) and vanadium (V) tells us something about the nature of charged particle reactions in stars: the increasing strength of the Coulomb barrier coupled with the maximum temperatures acheivable in the cores of stars determines the decreasing nature of the abundances in that region of the graph.
Synthesis of Nuclear Physics, Astronomy and Stellar Theory
Within the cores of stars -- in those gravitationally throttled nuclear furnaces -- is where the elements are forged. The task of experimental nuclear astrophysicist is to uncover those nuclear phenomena that influence the abundances observed in the plot above. This is done by contriving nuclear experiments targetted at measuring specific properties of nuclei, such as the spins, energy and lifetime of relevant excited states; atomic masses to high precision; nuclear reaction cross sections; and beta-decay lifetimes, to name a few. The targets of such experiments is guided by knowledge of the physical theory of stellar structure, novae and supernovae explosions, coupled with information arriving from our colleagues in the observational disciplines of gamma-ray and x-ray astronomy.
The experimental nuclear astrophysics group at TUM seeks to perform such experiments, here at our own tandem accelerator laboratory, in addition to international research facilities such as TRIUMF, GSI and RIKEN, to help shed more light on the questions of stellar nucleosynthesis, the structure of stars, and cosmic sources of the elemental abundances. In so doing, we learn more about our own origins; we each are deeply connected to these questions because we each are the by-products of steller explosions: we are, as stated by Carl Sagan, "star stuff".