Heterogeneous Oxidation Catalysis
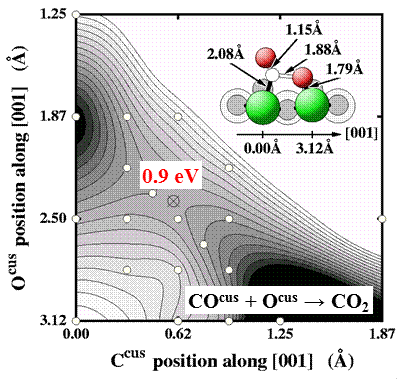
Partial and total oxidation reactions at metal and oxide catalysts play a key role in preeminent processes like contaminant removal (catalytic car converter) or the production of fine chemicals. The employed oxidizing environments, often comprising atmospheric or higher pressures of O2, may critically change the oxygen-load of the active surface and may, in fact, even induce morphological transformations between a metallic and an oxidic state of the catalyst surface. As this may in turn critically affect the obtained activity and selectivity towards desired products, heterogeneous oxidation catalysis is a paradigmatic reaction class highlighting the necessity to characterize and understand the catalytic function operando, i.e. during operation. While corresponding atomic scale information from experiment is only just emerging, computational theory is challenged by the requirement to explicitly account for the interaction with the ambient gas-phase, by the requirement to establish atomistic models for surface morphological transformations, or the large amount of heat released during the typically highly exothermic reactions. Current activities in this focus area center on CO oxidation, deNOx catalysis, and water gas shift.